HUMAC PRODUCTS
HUMAC® Natur AFM MycotoxiSorb
Characteristics
- What is HUMAC® Natur AFM MycotoxiSorb?
-
HUMAC® Natur AFM MycotoxiSorb is a 100% natural feed material with a high content of humic acids (+ 65%) intended for the detoxification of animal feed from mycotoxins, bacterial toxins, toxic metals, organic toxins such as dioxins, PCBs and others that commonly enter the body through feed. HUMAC® Natur AFM MycotoxiSorb is indigestible, therefore toxins bound to its structure are removed from the body via excretion. The active ingredient comes from a special mechanical activation of Leonardite, a natural substance of plant origin with high biological efficacy and itself acts as an organic detoxifying agent. The product is mechanically processed using our own so-called dual activation to increase its bioactivity. HUMAC® Natur AFM MycotoxiSorb is completely natural without the use of chemical additives in the production process. The product is GMP + FSA assured and is Kosher certified. HUMAC® Natur AFM MycotoxiSorb is used by mixing it in a feed mixture.
- What does HUMAC® Natur AFM MycotoxiSorb do?
-
Reduces the content of mycotoxins present in feed.
Reduces the level of toxins in the gastrointestinal tract of animals by preventing their absorption into the body at the molecular level.
Supports the immune system and activates metabolism.
Regulates intestinal microflora, stabilizes the pH in the digestive tract and has a prophylactic effect on the development of diarrhea and other digestive diorders.
Supplements the animals with mineral and trace elements in chelated form, which are easily absorbable by the body.
Strongly binds toxic heavy metals (Cd, Cr, Hg, Pb, As and others) and prevent their resorption in the body. It can also adsorb fluorides, organophosphates, chlorine-based insecticides, PCBs and dioxins.
Maintains a stable micro-climate. - For who is HUMAC® Natur AFM MycotoxiSorb?
-
HUMAC® Natur AFM MycotoxiSorb is intended for feed manufacturers who produce high quality compound feeds and special feeds produced for larger establishments engaged in the breeding of cattle, pigs, poultry and other species, which focus on processing large volumes of feed and look for ways to minimize the risk of mycotoxin contamination.
- How much HUMAC® Natur AFM MycotoxiSorb is needed?
-
For the effective detoxification of feed and animals’ organisms, a dosage of 0,3 – 0,5% is recommended which equals to 3 – 5 kg of the product for 1000 kg of feed. For cattle, 50 g per day per adult cow and 20 g per day per calve is recommended to meet the desired effect.
In case of other questions, please contact us.
- How is HUMAC® Natur AFM MycotoxiSorb delivered?
-
HUMAC Natur AFM MycotoxiSorb is supplied in 25 kg PE bags in quantities of 10, 20, 40 or 50 units per pallet.
- Why is HUMAC® Natur AFM MycotoxiSorb unique?
-
Unlike aluminosilic clays (bentonite, zeolite), which mainly form physical bonds with organic substances, HUMAC® Natur AFM MycotoxiSorb forms strong chemical bonds with organic substances.
- How does HUMAC® Natur AFM MycotoxiSorb work?
-
Due to the high content of humic substances (+ 65%), which have a complex organic structure with many different chemical groups and reaction sites, HUMAC® Natur AFM MycotoxiSorb can form strong chemical bonds with various organic substances and has the ability to bind various heavy metals.
Mycotoxins
- Mycotoxins in feed: a global challenge
-
The presence of toxins and contaminants in feed is a worldwide problem. The most common source of toxins are filamentous fungi, especially of the genera Fusarium Penicilium and Aspergilus, but also others, for example Claviceps, Alternaria, Sordaria Chaetomium. The secondary metabolites of these fungi are called mycotoxins (derived from the Greek word mycos, which means fungus), and from the Latin word toxicum, which means poison). According to a global 10-year mycotoxin study conducted by the Biomin Research Center in Austria, out of 74,821 samples of feed and feed materials collected from 100 countries, up to 88% of samples were contaminated with at least one mycotoxin (Gruber-Dorninger et al., 2019). Therefore, both the private and public sectors are making significant efforts to address the challenge of mycotoxins in feed in order to maintain production capacity and produce safe food. One of the most commonly used options is the elimination of mycotoxins directly in the feed using various absorbers.
Under favorable conditions, fungi begin to produce toxins after 5 days of infection. Mycotoxins can form before harvest (especially of the genus Fusarium) or during storage. Of the approximately 400 mycotoxins known so far, about a tenth significantly affect the economy and productive health of animal husbandry. The mycotoxicosis caused by these mycotoxins is diverse and includes a wide range of animal species, including humans. Mycotoxicosis can present itself with various clinical signs influenced by various factors such as the type and concentration of the toxin, the species, age and state of health of the animal. Long-term exposure, even at low doses, results in a state of increased susceptibility to secondary diseases of viral, bacterial and fungal origin. A mixture of different mycotoxins usually occurs in feeds, therefore the resulting toxic effect may be significantly worse than with individual toxins, e.g. the ever present DON stimulates the effects of several other mycotoxins.
- Where do mycotoxins most often occur?
-
Cereals (especially rye and triticale): fusariotoxins (trichothecenes), ergot alkaloids (ergotoxins)
Maize: citreoviridine, penicillic acids, aflatoxins, fumonisins, zearalenone
Silage: patulin, fumonisins, citrinin, cyclopiazonic acid
Hay, straw: T-2 toxin
Oilseeds, extracted meal and pomace: aflatoxins
Animal meal: zearalenone (F-2 toxin) - What problems do mycotoxins cause?
-
Acute poisonings by individual mycotoxins are very rare. After ingestion of high doses, clinically apparent symptoms lead to death.
Prolonged exposure may cause the following problems:
Aflatoxins (especially AFB1) - liver damage
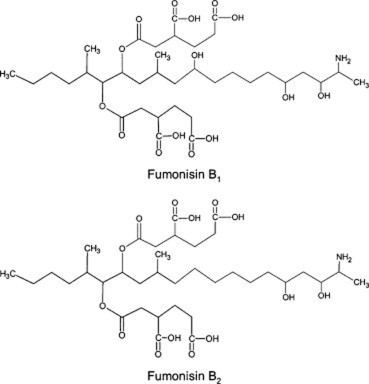
Fumonisins
- horse, donkey - ELEM leukoencephaloma
- pigs - PPE pulmonary edemaOchratoxins (OTA) - damage to the kidneys and liver
Zearalenone (F-2 toxin) - a strong estrogenic effect - reproductive disorders
Fusarochomanone - chickens, guinea pigs - TOP - tibial dyschondropathy - limb bone deformity
Ergot alkaloids (ergotoxins) - CNS disorders, convulsions, respiratory disorders, miscarriages
T-2 toxin - acute inflammation of the stomach and intestines - bloody diarrhea, bleeding in the skin and oral cavityThe causes of these problems are mainly due to the fact that almost all mycotoxins (all trichothecenes, ochratoxins and others) suppress the immunity of animals and they are subsequently more susceptible to diseases of viral, bacterial and fungal origin. Most often, multiple mycotoxins are present in feed at the same time due to their synergism, and otherwise non-toxic amounts can cause health problems that reduce the performance of animals, respectively specific clinical signs of a particular mycotoxin may occur, which would otherwise only appear at many times greater doses of the individual mycotoxin. The synergism of the ever present deoxynivaletol (DON), which potentiates the effect of aflatoxins, is well known. Different mycotoxins attack different organs and metabolic processes, resulting in a reduction in overall metabolism, apathy, reduced feed intake and a significant reduction in performance. In addition to feed, animals can be exposed to mycotoxins from litter. Stychybotriotoxin (T-2 toxin) spores are released from moldy straw (hay) - the only spores that contain the toxin that reach the lungs and skin by breathing.
With long-term intake of even small doses, other effects may also occur:
Carcinogenic: liver cancer - aflatoxins; kidney tumors - ochratoxins
Teratogenic and mutagenic: high embryonic mortality, stillborn and deformed pups - deoxynivaletol, patulin, zearalenone, fumonisins, alternariols. - How are mycotoxins removed from feed?
-
Physical-chemical methods (decomposition with calcium chloride, ammonia, hydrogen peroxide, ascorbic acid, or a combination of heat and pressure or ultraviolet radiation), adsorption with clay materials or activated carbon are used to detoxify feeds. The disadvantage of these methods is the considerable non-specificity of the degradation or binding of mycotoxins and the degradation of essential nutrients that can occur.
Most mycotoxins have a very stable chemical structure, which provides them with a high thermostability and resistance to decomposition during changes to pH or reactions with additives. It is therefore necessary to increase these parameters, which also increases the cost of detoxification.
Ultraviolet radiation can be very effective in degrading some mycotoxins, such as aflatoxins, but it also has a destructive effect on some nutrients in feed.
Sorbents are most often minerals capable of adsorbing or binding mycotoxin molecules, which subsequently cannot be absorbed and digested in the gastrointestinal tract. Activated carbon, synthetic zeolites and mineral clays are most commonly used. The effectiveness of sorbents depends on various factors - adsorption capacity, their molecular structure, purity, type of mycotoxin, etc. The disadvantage of these methods is that, depending on the type and intensity of the physical factor used, there is a different degree of disruption of nutrients and their absorption, thus reducing the nutritional value of the feed.
How does HUMAC Natur AFM Mycotoxicosorb work?
- Mycotoxins in feed: a global challenge
-
Humic acids are a natural heteropolymer material formed by the transformation of organic matter. Their molecular structure consists of a wide range of organic structures and functional groups such as aromatic structures and aliphatic chains, benzene nuclei, hydroxyl, carboxyl, ether, ester, carbonyl, amino groups and others. These impart characteristic chemical and microbiological properties to humic acids. The molecular weight of humic acids ranges from 20,000 to 150,000 Da, depending on the source and method of treatment. They are also ion exchangers of a reductive character and have amphoteric properties enabling them to bind ions by various mechanisms, both chemical and physical, as well as adjust the pH in the digestive tract.
Due to their large specific surface area and pseudomicellar structure with a large number of reactive chemical groups, they react with the functional groups of various toxic organic compounds such as mycotoxins, bacterial toxins, endotoxins, herbicides, persistent organic substances (PCBs and dioxins) and form chemical bonds. Humic acids have an affinity for toxic heavy metals (Cd, Cr, Hg, Pb, As and others) and prevent their resorption by chelating.
Humic acids can resist the digestive process in the gastrointestinal tract and are subsequently excreted from the body along with the bound toxins.
- Where do mycotoxins most often occur?
-
Humic acids are ion exchangers and can release the necessary microelements such as Fe, Cu, Co, Mn, Mo and a whole range of other trace elements.
They can bind certain viruses and prevent them from replicating.
They activate immunocompetent cells, thereby increasing the body's defenses.
They activate the production of anti-inflammatory cytokines that suppress inflammation.
They restore the electrolytic balance of cells (especially intestinal), thus protecting them from damage.
In the digestive tract, they support the development of beneficial microflora and suppress pathogenic microflora.
They have strong reductive properties, thus preventing undesired oxidation and the formation of free radicals, which they are able to eliminate.
They have good buffering capacity and maintain a suitable pH in the gastrointestinal tract.
Overall, humic acids prevent the resorption of toxic compounds, promote immunity and the development of beneficial microflora, suppress inflammation and ensure good functional health of animals. The result is improved production parameters: weight gain, milk yield, laying and improved reproductive performance (shortened service period, reduced the insemination index, increased number of litters and hatchability, development of more viable young) and thus reduce their morbidity and mortality. - What problems do mycotoxins cause?
-
Chemical interactions
Humic acids (HA) as a natural, heteropolymeric material, formed by the process of transformation of organic matter, naturally consists of a wide range of chemical structures and functional groups (aromatic structures and aliphatic chains, benzene nuclei, hydroxyl, carboxylic, ether, ester, amino groups and others). ), which give humic acids characteristic chemical as well as microbiological properties.
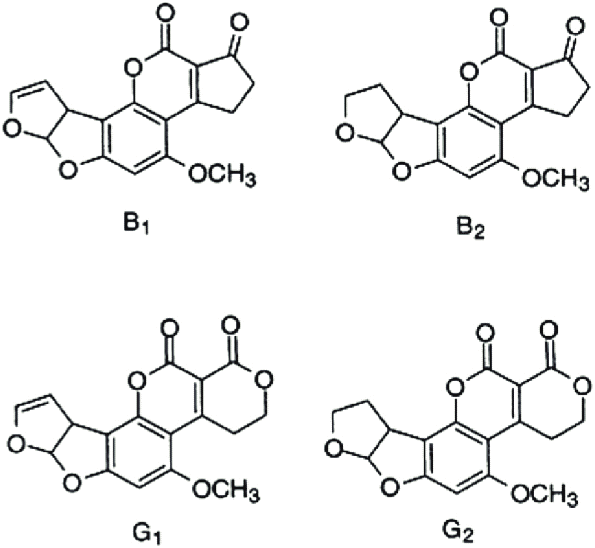
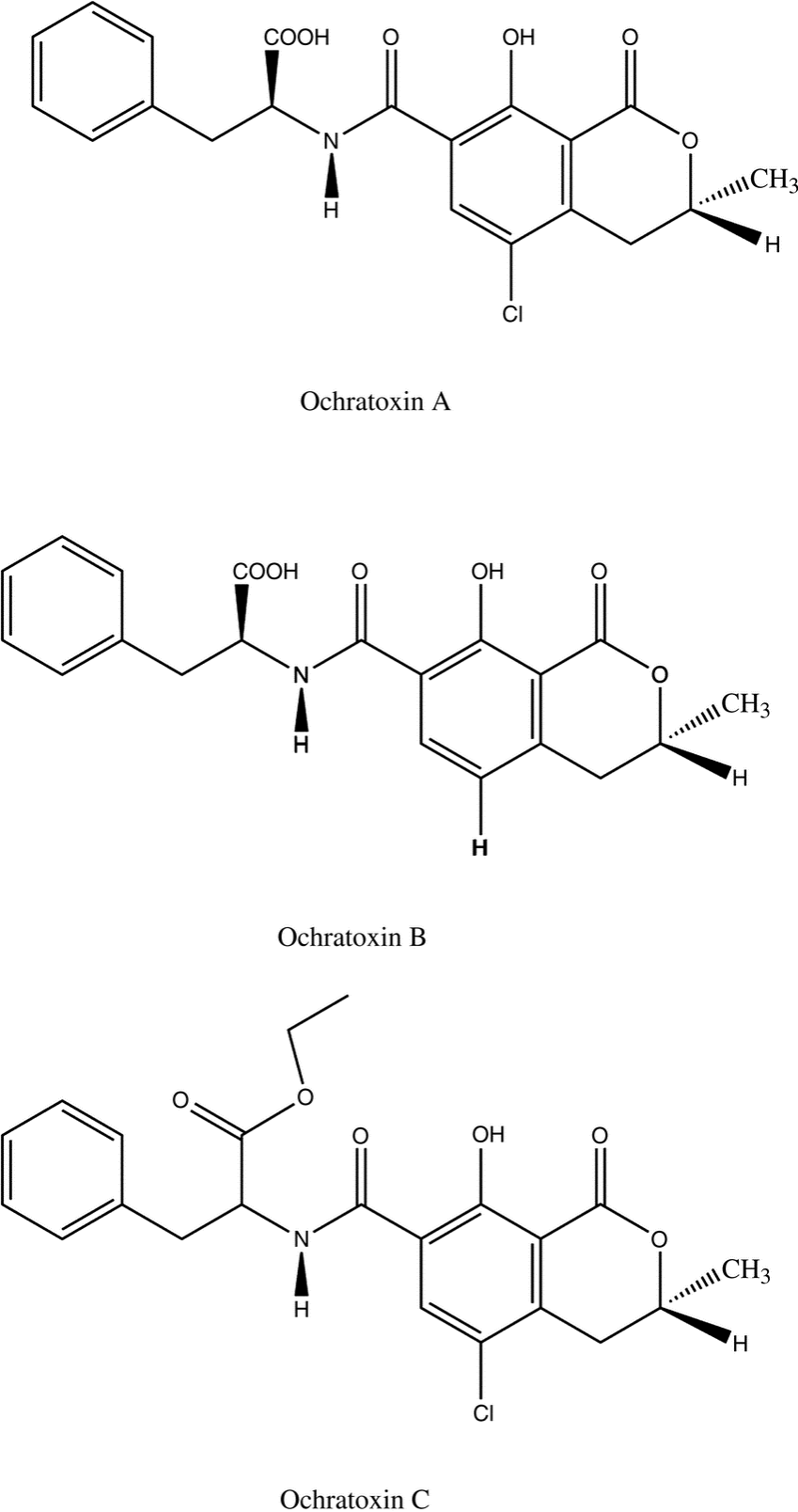
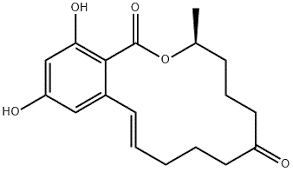
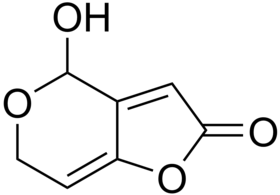
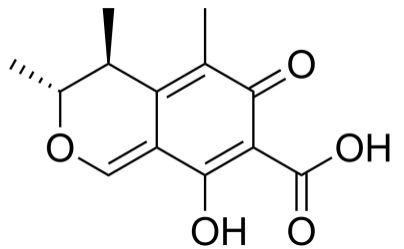
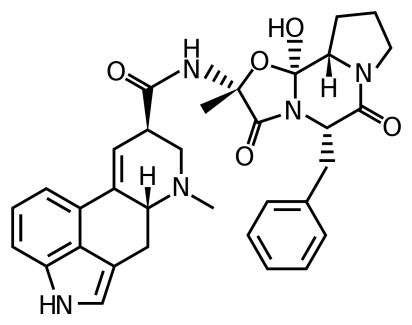
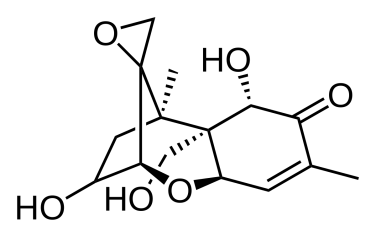
The most common mycotoxins such as aflatoxins, ochratoxins, zearalenone (ZEA) patulin, sterigmatocystins (STCs), citrinin, ergotamine, deoxynivaletol (DON), fumonisins, trichothecenes (Awuchi et al., 2021), also contain various functional groups similar to those in HA (in the pictures below).
The functional groups in HA and mycotoxins can interact with each other to form different bonds. The most common bond is a hydrogen bond with an energy of 10 - 40 kJ · mol-1, which can be formed by mutual interaction of acceptors (ketone, ether, ester, ...), donor (alkyl, ...) and donor-acceptor (hydroxyl, carboxyl) , amino, ...) groups. The energy of these interactions is multiplied by the number of bonds formed, and as a result, mycotoxins can be tightly bound to HA.
Thanks to aromatic structures, weaker π-π interactions (8 - 12 kJ · mol-1) of delocalized π electrons are also possible.
By reacting a carboxylic acid with an alcohol in an acidic medium, an ester bond can be formed (esterification). This covalent bond has an energy of 200-400 kJ · mol-1 and is therefore a sufficiently strong bond between mycotoxin and HA.
Due to the pseudomicellar structure of HA (Engebretson & Von Wandrusrka, 1994; R. L. Wershaw, 1994; Robert L. Wershaw, 1993), there is a hydrophobic region in the secondary structure of HA that can associate hydrophobic toxin molecules.
Through the synergy of several interactions, mycotoxins can be firmly bound to humic acids potentially forming resistant supramolecular structures.
References
Awuchi, C. G., Ondari, E. N., Ogbonna, C. U., Upadhyay, A. K., Baran, K., Okpala, C. O. R., Korzeniowska, M., & Guiné, R. P. F. (2021). Mycotoxins affecting animals, foods, humans and plants: Types, occurrence, toxicities, action mechanisms, prevention and detoxification strategies-a revisit. In Foods (Vol. 10, Issue 6, p. 1279). Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/foods10061279
Engebretson, R. R., & Von Wandrusrka, R. (1994). Microorganization in Dissolved Humic Acids. Environ. Sci. Technol, 28, 1934–1941.
Gruber-Dorninger, C., Jenkins, T., & Schatzmayr, G. (2019). Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, Vol. 11, Page 375, 11(7), 375. https://doi.org/10.3390/TOXINS11070375
Wershaw, R. L. (1994). Membrane-micelle model for humus in soils and sediments and its relation to humification. US Geological Survey Water-Supply Paper, 2410.
Wershaw, Robert L. (1993). Model for Humus: In Soils and Sediments. Environmental Science and Technology, 27(5), 814–816. https://doi.org/10.1021/es00042a603